1.Introduction
Plants produce a large number of primary and secondary metabolites. Primary metabolites are molecules that are directly related to the plant’s vital functions, while secondary metabolites are organic molecules that fulfil non-essential functions in the plant. These include defending against attacks by pathogenic microorganisms, rebuilding and reinforcing cell walls, establishing flower and fruit colour, contributing substantially to flavours and odours, acting as cell antioxidants, attracting pollinators, favouring fruit set, absorbing UV radiation, repelling herbivores, and so on.
In particular, the secondary metabolites of plants include polyphenols or phenylpropanoids, which are widely distributed in the plant kingdom and can be biochemically modulated. The polyphenol content varies from plant to plant depending on the species or variety. Scientific research confirms their positive physiological effects on human health, in the prevention and treatment of diseases, and their involvement in food quality.
Most existing phenolic compounds are synthesised in two fundamental metabolic pathways in plants, the shikimic acid pathway and, to a lesser extent, the malonic acid pathway. The output of the shikimic acid pathway are the aromatic amino acids phenylalanine, tryptophan and tyrosine. Most phenolic compounds are derived from phenylalanine.
The enzyme phenylalanine ammonium lyase (PAL) is responsible for the synthesis of polyphenols in plants and is located at a branch point between primary and secondary metabolism. The essential amino acid phenylalanine is part of the primary metabolism of plants and enters secondary metabolism when the enzyme PAL catalyses the elimination of ammonium, converting phenylalanine into cinnamic acid, a precursor of simple phenols such as coumaric and benzoic acids, as well as complex phenols such as flavonoids, phytoalexins, lignins, suberins, stilbenes, and others.
Plants defend themselves against pathogen infections through by synthesising substances known as phytoalexins (post-infection). These substances are toxic to a broad spectrum of pathogens. In contrast, phytotoxins, are preinfectional toxic substances. These are constituent compounds of plants and are present in healthy tissues in various concentrations to help protect them from possible infection. Both substances are naturally synthesised by PAL, although this activity increases considerably when there is potential damage caused to the plant by a pathogen or a situation of biotic stress, or when a preventive defensive mechanism is triggered through the application of an elicitor product.
The goal of this study was to determine the effect that treatments with Brotomax® had on the activity of the PAL enzyme, which is responsible for synthesising polyphenols in plants and, among other functions, favouring the formation of lignin, preventing the infection penetrating the plant tissue, as well as inducing the synthesis of flavonoids with antioxidant properties, thanks to their capacity to inhibit the production of free radicals.
2. Materials and methods
The experiment was carried out in a commercial plantation of black seedless table grapes (Vitis vinifera) of the “Melody®” variety. The vines were 6 years old and had a planting frame of 3.50 x 2.90 m; the vineyard was under netting and plastic and located in the town of Hoya del Campo (Murcia). The irrigation system had emitters every 75 cm within each line and a flow rate of 2-4 L/Ha.
The experimental design was a randomised block design, with four replicates for each treatment (T1: Brotomax® via irrigation at a rate of 6 L/Ha; T2: Brotomax® via foliar application at a rate of 3 L/Ha; T3: Control – Untreated). Each block was divided into three sub-plots of 3.50 m x 39 m (10 plants/plot).
Treatment 1 (T1) was applied via irrigation and Treatment 2 (T2) was applied onto the leaves on 02/05/19, at phenological stage “J”, i.e., fruit set. The weather conditions were typical for the time of year and area.
21 days after application, 24 leaves were taken at random from plants in each treatment and repetition, with a homogeneous sample being obtained from the different treatments applied. These samples were transported in cold storage to the CSIC Sevilla (CSIC) – Instituto de la Grasa where, after being received, they were kept frozen at -20°C until their analysis.
From each sample, 4 g (in duplicate) were used to obtain the enzyme extract (Figure 1), with four measurements being taken from each sample. The results are expressed as the arithmetic mean ± standard deviation for the different determinations.
To correctly determine this enzyme activity, the first step is to obtain extracts that contain the enzymes in their native form, avoiding alterations during the extractive process. For this purpose, a buffer solution was used, at the appropriate pH, and the samples and reagents were kept at a low temperature (in an ice bath and/or liquid nitrogen) throughout all the stages of obtaining and purifying the extracts.
Once the corresponding extracts had been obtained, the CSIC research team followed the protocol described below (Figure 2), which consists of bringing the enzyme present in the extract into contact with a specific substrate, then using a spectrophotometer to determine the increase or decrease in absorbance that occurs as a result of the action of the enzyme on the substrate at the specific wavelength for each reaction. The units of PAL enzyme activity are calculated as a function of these changes in absorbance per 1 g of plant material per minute.
3. Results and Discussion
The action of Brotomax® as an elicitor has been demonstrated in several species of agricultural interest. In citrus, it is well known to stimulate the biosynthesis of flavanones and flavones, as well as the expression of the phytoalexin scoparone in infected fruit in Nova Tangelo, resulting in increased resistance to attack from Phytophtora. In olive trees, it has been found to influence the synthesis of phenolic compounds that increase the antioxidant and antifungal potential of different varieties.
In cotton, it increases phenolic content, chlorophyll, and ethylene production, which directly contribute to an increase in the natural defence mechanisms of cotton plants against Fusarium oxysporum. In vines, the total polyphenol content in the different organs increases, and there is less obstruction of xylem vessels in the newly formed stems and roots. There are also studies on the increase of the phenolic composition in the skin, must and pips. In strawberry, it has been shown that genes related to plant defences are activated, inducing polyphenol production and increased resistance to Colletotrichum acutatum.
All this research shows that the Brotomax® product increases the levels of phenolic compounds in the plant, so it is interesting to determine whether this increase in phenylpropanoids is related to an increase in the enzymatic activity of PAL, a complex and versatile enzyme whose functions include regulating the metabolism of phenolic compounds, including resveratrol, lignins, suberins and cinnamic acid derivatives, all of which are related to the plant’s defence mechanisms.
In this study, a statistical analysis was carried out to check the existence of significant differences between the three treatments for PAL enzyme activity, using a one-factor analysis of variance (ANOVA). This resulted in the rejection of the null hypothesis of equality of the means of the three treatments.
Fisher’s LSD (Least Significant Difference) test for multiple comparisons was applied, which allowed us to compare the means of the three treatments with one another. As a result, it was found that there were statistically significant differences between the different treatments (p<0.05), with Treatment 1 (T1) showing the greatest difference with respect to Treatment 3 (T3). The results obtained are shown in Figure 3.
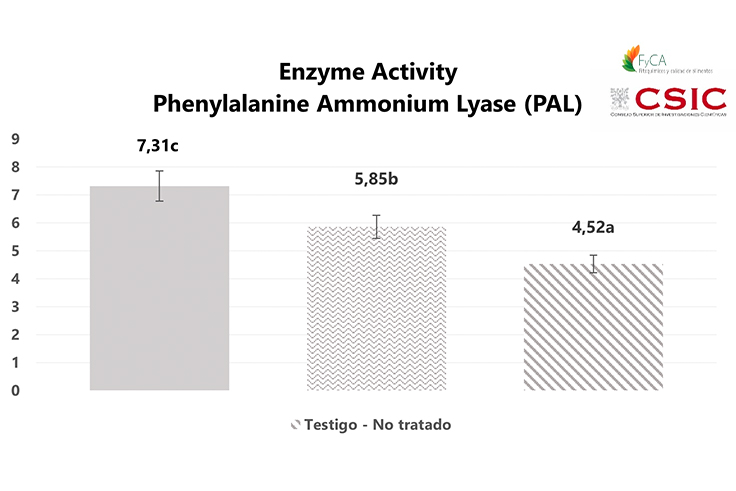
The results obtained show that Treatment 1 (T1) applied via irrigation at a rate of 6 L/Ha with Brotomax® achieved better results than Treatment 2 (T2) applied to the leaves at a rate of 3 L/Ha, although both treatments presented greater PAL enzyme activity than the untreated or control plants (T3), with significant differences between the three treatments.
4. Conclusions
Brotomax® is an elicitor capable of considerably increasing the activity of the PAL enzyme, with the greatest increase in activity occurring when the treatment is applied via irrigation.
Brotomax® does not directly supply polyphenols to the plant. Instead, it influences the biochemical modulation of these compounds, leaving nature to regulate and balance their action.
References
Alain-M., B. Lignins and lignification: Selected issues. Plant Physiol. Biochem. 2000. 38: 81-96.
Ávalos-García, A. y E. Pérez-Urria. 2009. Metabolismo secundario de plantas. Reduca (Biología) Serie Fisiol. Veg. 2, 119-145.
Berra, B., Caruso, D., Cortesi, N., Fedeli, E., Rasetti, M.F. y Galli, G. 1995. Antioxidant properties of minor polar components of olive oil on the oxidative processes of colesterol in human LDL. Rivista Italiana Sost Grasse. 72, 285-291.
Botía, J.M., Fuster, M.D., Porras I., García Lidón A., Sabater F., Ortuño A., Del Río J.A. 1995. Brotomax: un posible modulador de la expresión de flavonoides en cítricos. Levante Agricola 34, 44-47.
Botía, J.M., Fuster, M.D., Porras I., García Lidón A., Ortuño A., Lacasa A., Del Río J.A. 1997. Efecto de Brotomax sobre los procesos de resitencia en frutos de tangelo Nova frente al ataque de Phytophtora parasítica. 1997. Levante Agricola, 36, 67-68.
Botía, M., Ortuño, O., Benavente-García, Báidez A.G., Frías J, Marcos, D., Del Río J.A. 2000. Efecto del Brotomax sobre el crecimiento, rendimiento en aceite y expresión de compuestos fenólicos en frutos de Olea europea L. (Variedad Picual y Villalonga). Mercacei 20-26/03, 16-19.
Clifford MN. 2004.Diet-derived phenols in plasma and tissues and their implications for health. Planta Med 70, 1103-1114.
Clifford, M.N.,1992. Sensory and dietary properties of phenols. Proceeding of the 16th international conference of grape polyphenol. 16 (11): 18-23.
Dewick, P. M. 2002. Medicinal natural products: a biosynthetic approach: John Wiley & Sons.
Del Río Conesa, J.A., Gómez López, P., González Baidez, A., Ortuño Tomás, A.M., García, G., Frías, V., Aguado, A. 2004. Mejora de la germinación, vigor y resistencia frente a infecciones por Fusarium oxysporum en plantas de algodón por tratamientos con Brotomax. Phytoma 157, 54-59.
Del Río J.A., Ortuño A., Botía, J.M., Frías, V. 1998. Influencia del Brotomax sobre la síntesis de compuestos fenólicos y su relación con el potencial antifúngico y antioxidante en Olea europaea. Phytoma 102, 150 – 153.
Dixon, R. A., Pavia, N. L. 1995. Stress- induced phenylpropanoid metabolism. Plant Cell 7, 1085- 1097
Fuster, M.D., Porras I., García Lidón, A., Sabater, F., Ortuño, A., Del Río, J.A. 1994. Efecto del Brotomax sobre el crecimiento y producción de flavonoides en frutos de tangelo Nova. Levante Agrícola, 33, 60-64.
Fuster, M.D., García Puig, A., Porras I., García Lidón A., Sabater F., Botía, J.M., Ortuño A., Del Río J.A. 1995. Selection of citrics highly productive in secondary metabolites of industrial interest. Modulation of synthesis and/or accumulation processes. Current Trends in Fruit and Vegetables Phytochemistry, 81-85.
Gutierrez, J.C. et al. 2000. Estudio del efecto de Brotomax sobre la tolerancia a Verticillium dahliae en el cultivo del algodón upland Gossypium hirsitium L en Andalucía. Phytoma 122, 144 -148.
Jiang,F. y Dusting,G.J. 2003. Natural phenolic compounds as cardiovascular therapeutics: potential role of their antifinflammatory effects. Current Vascular Pharmacology. 1 (22), 135-156.
Muñoz Blanco, J., Caballero J.l., Garrido Gala, J., Soliveri, J., Copa Patiño, J.L., Martinez Ros, J.M., Rodriguez Olmos, A., Frías, V., Perdices Hoyo, M. 2014. Natural elicitors of plant defense response in strawberry. Journal of Berry Research 4, 37 -45.
Muñoz Blanco, J., Caballero J.L., Soliveri, J., Copa Patiño, J.L., Martinez Ros, J.M., Rodriguez Olmos, A., Frías, V., Perdices Hoyo, M. 2014. Natural elicitors of plant defense response in strawberry. Freshuelva 27.
Posada Jaramillo, M., Pineda-Salinas, V. y Agudelo- Ochoa, G. M. 2003.Los antioxidantes de los alimentos y su relación con las enfermedades crónicas.
Quiñones M., Miguel M., Aleixandre A. The polyphenols, naturally occuring compounds with beneficial effects on cardiovascular disease. Nutr. Hosp. Vol 27 no 1, Madrid Jan/Feb.2012.
Sija, S.L.,Potty, V.P., Santhoshlal, P.S. 216. Detection of phenylalanine ammonia-lyase activity in different plant parts of Anacardium occidentale L. Int J Pharm Bio Sci 7(4), 100 – 104.
Sirin, S., Bbaoglu, Aydas S., Aslim, B. 2006. Biochemical evaluation of Phenylalanine Ammonia Lyase from Endemic Plaant Cyathobasis fruticula (Bunge) Aellen. For the Dietary Treatment of Phenylketonuria. Food Technol. Biotechnol 54 (3) 296–303.
Sgarbi, E., Baroni Fornasiero, R., Paulini Lins, A., Medeghini Bonatti, P. 2003. Phenol metabolism is differentially affected by ozone in two cell lines from grape (Vitis vinífera L.) leaf. Plant Science 165, 951-957.




